Technologies
Size-Exclusion Chromatography (SEC)/Gel Permeation (GPC)
It is a technique that separates macromolecules (polymers) based on their hydrodynamic size in solution. The relative molecular weight (molar mass) of an unknown polymer can be determined by calibrating the GPC column set by injecting a series of narrow polymer standards, and subsequently comparing elution volumes. If the GPC system uses advanced detectors such as a light-scattering detector, calibration of the detector itself will provide the absolute measurement of molecular weight.
-
- Short review:
- Liquid chromatographic technique that separates macromolecules based on their size
-
- Required state:
- Solid or solution
-
- Sample amount:
- > 50mg
-
- Limit of detection:
- 5 µg/mL
-
- What it provides:
- Mn, Mw, Mz, Đ, Rg, RH, IV, branching, dn/dC, dA/dC, concentration
-
- Variations
- GPC-MALS, preparatory/semi-prep SEC, high-temperature SEC
Liquid Chromatography Time of Flight Tandem Mass Spectrometry (LC-MS)
like conventional HPLC, separates molecules based on the affinity to the stationary phase of the column. Following separation in the LC column, the resolved sample enters the mass spectrometer which provides an accurate molar mass measurement for each molecule and/or fragment.
-
- Short review:
- Liquid chromatographic technique that separates molecules based on their affinity to the stationary phase, followed by electrospray ionization mass spectrometry (ESI)
-
- Required state:
- Solid or solution
-
- Sample amount:
- > 5 µg
-
- Limit of detection:
- 1 ng/mL
-
- What it provides:
- m/z, molar mass, concentration, polarity, structural information

Fourier-Transform Infrared Spectroscopy (FT-IR)
A spectroscopic technique used to identify the presence or absence of certain functional groups on a material by measuring the resonance of covalent bond frequencies. These functional groups include carbonyl, hydroxyl, carboxyl, aliphatic, amine, amide, alkenes, and more.
-
- Short review:
- A spectroscopic technique which identifies functional groups based on covalent bond resonance
-
- Required state:
- Solid
-
- Sample amount:
- > 1mg
-
- What it provides:
- Functional group information
Nuclear Magnetic Resonance Spectroscopy (NMR)
Provides a detailed description of the chemical structure of a molecule by identifying the quantity and chemical environment of a predetermined atom such as hydrogen or carbon. The most popular technique is 1H-NMR, which measures the atomic resonance of protons, and thus can elucidate the complete chemical structure of any organic molecule containing hydrogen. 13C-NMR measures carbon-13 resonance and 2D-NMR provides a more thorough investigation in order to determine structural characteristics.

-
- Short review:
- A spectroscopic technique which uses the resonance of particular nuclei to elucidate the chemical structure of a molecule
-
- Required state:
- Solid or solution
-
- Sample amount:
- > 10mg
-
- What it provides:
- Mn of polymers, chemical structure, number of protons
-
- Variations:
- Proton NMR (1H-NMR), Carbon NMR (13C-NMR), solid state NMR, 2D-NMR

Differential Scanning Calorimetry (DSC)
Measures thermal properties by applying heat to a polymer, and measuring the resultant response and heat capacity. DSC provides the glassy transition temperature (Tg), melting temperature (Tm), and crystallization temperature (Tc), all key properties of polymeric materials.
-
- Short review:
- A thermal technique used to investigate the polymers response to heating
-
- Required state:
- Solid
-
- Sample amount:
- > 10mg
-
- Sample amount:What it provides:
- g, Tm, Tc, heat capacity, ∆H, crystallinity
Thermogravimetric Analysis (TGA)
like DSC, is also a thermal analytical technique, but it can heat the sample until complete combustion. TGA has the potential to measure polymer purity (by vaporizing all solvents), moisture content, volatiles content, thermal stability, and decomposition kinetics.

-
- Short review:
- A thermal technique which applies heat to a sample and to measure its purity and degradation process
-
- Required state:
- Solid
-
- Sample amount:
- > 10 mg
-
- What it provides:
- Purity, moisture content, thermal degradation profile
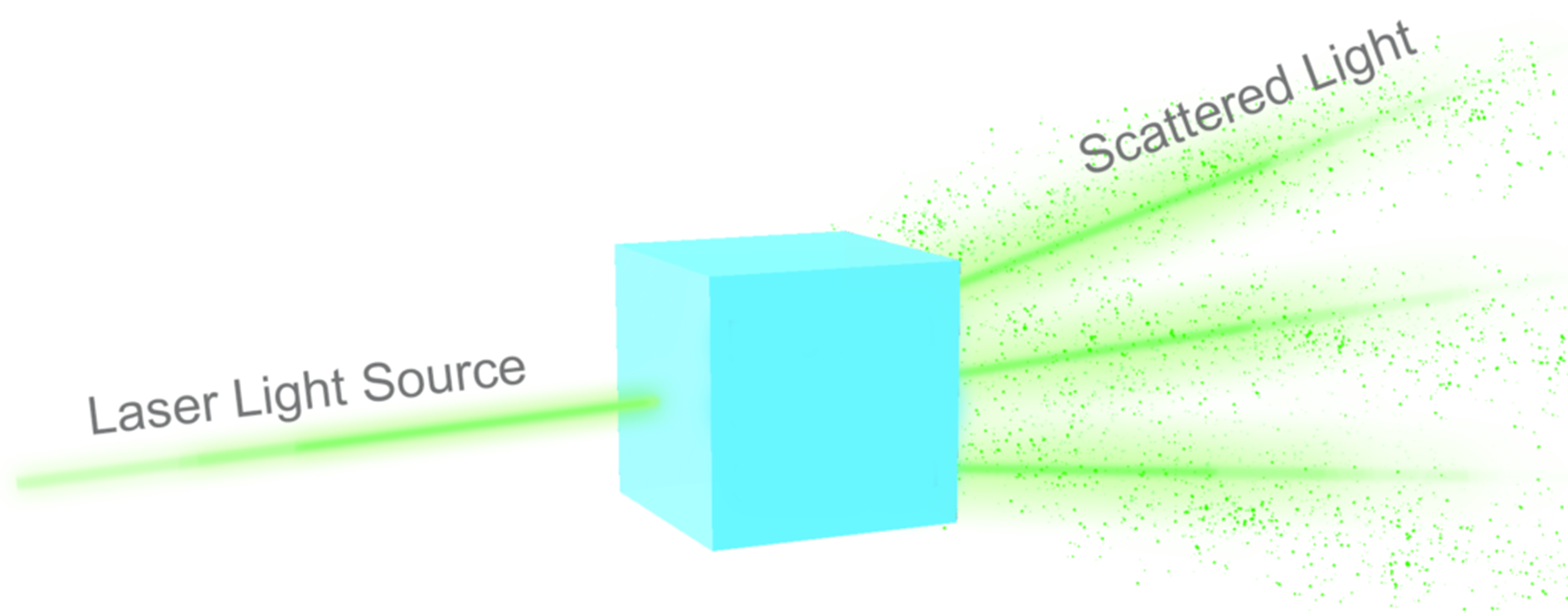
Dynamic Light Scattering (DLS)
A physical characterization technique used to measure the average size of nanoparticles suspended in solution by irradiating with a laser and measuring the Brownian motion of the particles in solution through scattered light. This technique may also measure the zeta potential (charge) of a colloid, which is an indirect measurement of its stability in solution.
-
- Short review:
- A light-scattering technique which measures the size and shape of a suspension of nano- or micro-particles
-
- Required state:
- Suspension in solvent
-
- Sample amount:
- <1 mg/mL
-
- What it provides:
- Zeta potential, average size (nm)
X-Ray Diffraction (XRD)
A technique used for phase identification of a crystalline material and can provide information on unit cell dimensions. The analyzed material is finely ground, homogenized, and average bulk composition is determined. It provides the average crystallite size and the ratio of crystalline vs amorphous phases in a polymer.

-
- Short review:
- A technique which probes a solid’s crystal structure
-
- Required state:
- Solid
-
- Sample amount:
- > 50 mg
-
- What it provides:
- Crystallinity information

Dilute Solution Viscometry (DSV)
Used to measure a polymer’s intrinsic viscosity which is a measure of the change in solvent viscosity by the presence of polymer solutes in specific solvents and temperatures. Based on the measured intrinsic viscosity of a polymer, the viscosity average molecular weight and hydrodynamic radius of polymers can be also be calculated.
-
- Short review:
- A bulk solution technique which measures the polymer’s impact on the viscosity of the solution
-
- Required state:
- Solution
-
- Sample amount:
- <1 mg/mL
-
- What it provides:
- Intrinsic viscosity, inherent viscosity
Scanning Electron Microscopy (SEM)
A technique that produces detailed, high-resolution images of a sample by emitting a focused electron beam at the surface of the sample. The electrons interact with the atoms of the sample and thus provide surface topography information and the size and surface shape of any present nanoparticles.
-
- Short review:
- A high resolution microscopic technique which produces detailed images of particles and surfacesLiquid chromatographic technique that separates molecules based on their affinity to the stationary phase, followed by electrospray ionization mass spectrometry (ESI)
-
- Required state:
- Solid
-
- Sample amount:
- > 10mg
-
- What it provides:
- Images, size
-
- Variations:
- SEM-EDX (energy dispersive X-ray spectroscopy)
X-ray Photoelectron Spectroscopy (XPS)
Known as Electron Spectroscopy for Chemical Analysis (ESCA), is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition, empirical formula, chemical state, and electronic state of the elements that exist within a material. The material can be a powder or a film deposited on a substrate. In a typical XPS experiment, a material is irradiated with a beam of X-rays and the resulting kinetic energy and number of electrons that escape from the top surface layer (usually 1 to 10 nm) of the material are detected. The emitted photoelectron has a characteristic kinetic energy (KE), which can be converted to the binding energy (BE) of the electron by the following equation:
KE = hʋ – BE – Φ. Where Φ is work function of the instrument.
The characteristic binding energies for each element are known and are used to identify the elements present on the surface of the sample.
-
- Short review:
- A quantitative surface-sensitive spectroscopic technique that identifies the elements that exist within a material as well as their chemical state, and the overall electronic structure and density of the electronic states in the material
-
- Required state:
- Solid
-
- Sample amount:
- > 10mg
-
- What it provides:
- Elemental composition, electronic structure, electronic state